Unlocking New Molecular Possibilities: Evolving Ribosomes
Written on
Chapter 1: The Ancient Origins of Ribosomes
Around four billion years ago, a primitive single-celled organism floated in the primordial waters of early Earth. This ancient life form already possessed many characteristics of contemporary organisms, including a functional ribosome—the remarkable, nanoscale machinery responsible for synthesizing the diverse proteins that sustain cellular life.
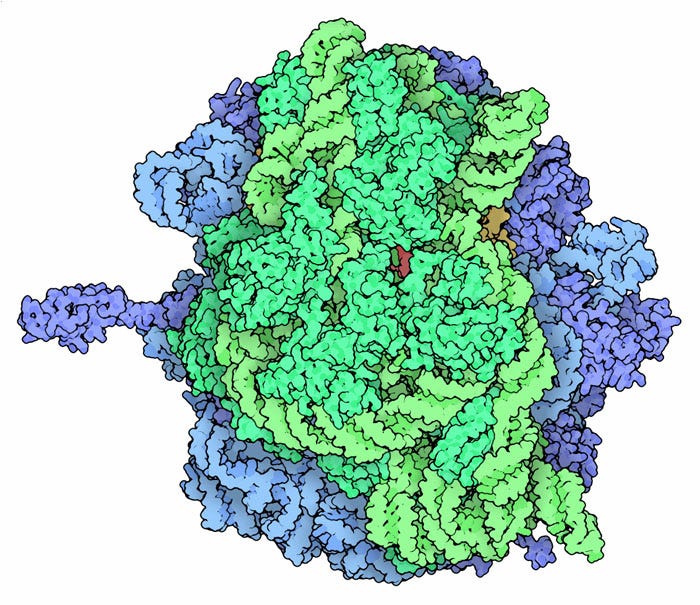
The ribosome is an extraordinary macromolecular machine that interprets mRNA sequences to create proteins. It performs a crucial role in a process called translation, where two distinct ribosomal subunits—the large and the small—work together to decode mRNA messages. The ribosomal RNA within these subunits drives their catalytic functions: a specific RNA strand in the large subunit facilitates the formation of peptide bonds between amino acids, while the small subunit contains RNA that identifies the starting point on the mRNA for translation. This process is so intricate that it has remained largely unchanged for nearly four billion years, and it took scientists decades to unravel its complexities. The discovery of the ribosome's structure and its interactions with various cellular components led to the awarding of the 2009 Nobel Prize in Chemistry to Venki Ramakrishnan, Thomas Steitz, and Ada Yonath.
Since the ribosome's identification, researchers have aimed to modify it, enhancing its ability to synthesize not only proteins but also novel types of molecules. This endeavor has encountered significant challenges due to the ribosome's highly conserved nature—altering its structure even slightly could be fatal to the organism. As a result, attempts to evolve the ribosome, particularly the peptidyl transferase center, have faced difficulties, as changes in this area often disrupt the interaction between the large and small subunits. However, groundbreaking progress has now been made.
Section 1.1: A Breakthrough in Ribosome Engineering
Researchers from Cambridge University have made a significant advancement by developing the first ribosomes capable of synthesizing polymers—a feat previously unattainable by natural ribosomes throughout cellular evolution. The findings were published in the December issue of Nature.
By linking the ribosomal RNA sequences from the large and small subunits together through a process called ‘stapling’, the team succeeded in creating ribosomes that could interpret mRNA messages without needing separate subunits. This experiment was complex, as many of the tested linkers rendered the ribosomes inactive. However, one variant exhibited high activity, allowing the two subunits to be functionally combined into a single structure.
Subsection 1.1.1: The Evolutionary Process
Through a technique known as directed evolution, the team refined the stapled ribosomes—an iterative process that gradually modifies the structure. This methodology, pioneered by Frances Arnold, who received the 2018 Nobel Prize in Chemistry, played a crucial role in their success.
The researchers further advanced their project by introducing the evolved, stapled ribosomes into living cells, completely replacing the natural ribosomes. This marks a historic milestone as it represents the first instance of an organism being created without any natural ribosomes—a notion previously deemed impossible due to the highly conserved nature of translation. The outcome was the generation of functional, semi-synthetic organisms capable of utilizing the new ribosomes to construct polymers not found in nature, including proteins with highly repetitive proline sequences.

Chapter 2: Implications of Engineered Ribosomes
Engineered organisms are already responsible for producing many familiar medicines, such as insulin and erythropoietin, which is used to treat anemia and kidney disease by stimulating red blood cell production. Jason Chin, a professor at Cambridge University's Laboratory for Molecular Biology and the leader of the research team, envisions utilizing these modified ribosomes to develop synthetic organisms that can produce advanced nanomaterials and pharmaceuticals. Because the ribosome interprets mRNA messages—messages that scientists can modify quickly—the engineered ribosomes could potentially be programmed to create an almost limitless array of polymers.
Despite the ribosome's long-standing unchanging status, the creation of designer versions marks a significant leap forward, opening new avenues for scientists eager to harness this molecular machinery for innovative applications.
Sources:
Schmied, W.H. et al. Nature 564, 444–448 (2018).
Ban, N. et al. Science 289, 905–920 (2000).
Clemons, W.M. et al. Nature 400, 833–840 (1999).
Fox, G.E. Cold Spring Harb. Perspect. Biol. 2, a003483 (2010).